Биосорбция свинца и меди при помощи апельсинового и лимонного пилинга
Ал Кадхими Зинах Али Хассон,
доктор биологических наук. Багдадский университет, Ирак.
Biosorption of lead and copper by using orange and lemon peels
Zinah Ali Hassoon,
Republic of Iraq. Ministry Of Higher Education and Scientific Research. Office: Reconstruction and Projects. University of Baghdad.
Intorduction: Environmental pollution
The environmental pollution is a phenomenon of great concern worldwide (Khan et al., 1996). Any change in physical, chemical or biological properties of water in a way leaves it unavailable can be defined as water pollution (Kannan, 1995). Heavy metal pollution represents an important environmental problem due to its toxic effects to the environment. The main sources of water pollution with various heavy metals are industrial effluents (Vijayaraaghavan et al., 2005).Heavy metals have a harmful effect on human physiology and other biological systems when they exceed the normal levels (Kobya et al., 2005),,so there is an urgent need to treat these toxic metals particularly in wastewater effluents before they are discharged into freshwater bodies (Ahalya et al., 2005). Various treatment technologies have been developed for the decontamination of water and wastewater carrying heavy metals. The most commonly used methods (Demirbas, 2008) are: Activated carbon adsorption, Chemical precipitation and Ion-exchange these methods have disadvantages of high cost and partial removal of certain ions (Rengaraj et al., 2001).Therefore, the search for efficient, ecofriendly and cost effective remedies for wastewater treatment has been initiated (Prasad and Freitas, 2003). In recent years, attentions have been focused on possible biological methods for the removal of heavy metals from industrial wastewater (Cho and Kim, 2003).
Biosorption has been proposed as one of the most promising biological techniques for the removal of toxic metals from wastewater. Biosorption has many advantages compared with those conventional techniques and these are: Less expensive, High efficiency,Metal selective, No sludge generation and Metal recovery(Volesky, 2007).
The ability of inexpensive agricultural residues to adsorb heavy metal ions has received considerable attention for the development of efficient techniques for wastewater treatment (Montanher et al. 2005). Such as Citrus peels, peel residue is composed mainly of water 80%, the remaining 20% solid fraction consists of pectin, soluble sugars, cellulose, proteins, phenolics, (Cameron et al., 2008).
Materials and Methods: Industrial wastewater samples
Examined industrial wastewater samples were collected from pasting unit wastewater before wastewater treatment unit, Each sample was subjected for the chemical and physical analysis such as Temperature, pH, Electrical conductivity and Lead and copper evaluation in industrial wastewater samples (APHA, 2005).
Biosorption experimental procedure:
Orange and lemon peels were collected and washed thoroughly using DDW, left to dry and powdered and sieved by 1.18mm. stainless steel sieves and used subsequently in experiments. All samples were stored and used for biosorption tests (Saikaew et al., 2009).
System design laboratory
The system was designed and the basis of its work depends on the use of citrus peels, the system consists of as shown in figure 2:1
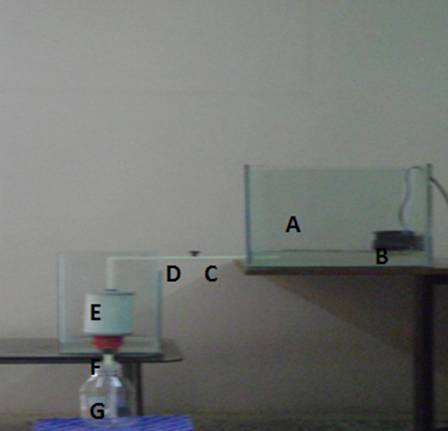
Figure 2-1: System design. A-Rectangular tank (18 liters),B-Mixer ,C-Pipeline deliver,D-Valve, E-Square tank (12 liters), F-Filter, G-Glass bottle.
2.5 liters of industrial wastewater was placed in tank A and 12.5 g of citrus peels was added to the tank and still about 1hour then the industrial wastewater transferred through pipeline deliver to the filter in tank E,then the water gathered in glass bottle for heavy metals concentration measurements by using flame atomic absorption spectrophotometer (FAAS).
Results: Chemical and physical measurements
Study results showed that the mean temperature values were ranged from 28.0±0.2 °C to 33.5±0.5 °C for the samples from pasting unit wastewater in State Battery Manufacturing company (SBMC) (Babyl 2 factory).
While the pH values of samples were ranged from 7.48±0.61 to7.95±0.22, it was found that the means of electrical conductivity values of samples were lying from 0.7±0.26 to 1.2±0.21 µ.S/cm.
The range of lead and copper values of wastewater samples were varied from 3.88±0.81 to 5.7±0.22 mg/L and from 0.9±0.21 to 1.4±0.21mg/L for lead and copper respectively.
The efficiency of laboratory design in lead and copper biosorption from industrial wastewater of SBMC:
The efficiency of laboratory design was tested by using lemon peels because of thier high biosorption capacity, the laboratory design showed high efficiency in lead and copper biosorption,the biosorption were 97.9±0.01% and 99±0.005% for lead and copper respectively.
References
1. Ahalya, N.; Ramachandra, T.V. and Kanamadi, R.D. 2005. Biosorption of Chromium (VI) from aqueous solutions by the husk of bengal gram (Cicer arientinum). Elec. J. Biotechnol.18 (3):44-48.
2. American Protection Health Agency (APHA), 2005. Standard methods for examination of water and waste water, 21st Ed., Washington.
3. Cameron, R.G.; Luzio, G.A.; Goodner, K. and Williams, M.A.K. 2008. Demethylation of a model homogalacturonan with a salt-independent pectin inethylesterase from citrus: I. Effect of pH on den-tethylated blocks size, blocks number and enzyme mode of action. Carbohydrate Polymers 71(2):287-299.
4. Cho, D. H. and Kim, E. Y. 2003. Characterization of Pb+2 biosorption from aqueous solution by Rhodotorula glutinis bioprocess. Biosyst. Eng. 25: 271-277.
5. Demirbas, A. 2008.Heavy metal adsorption onto agro based waste materials: A rev.J. Hazard. Mater. 157: 220-229.
6. Kannan, K. 1995. Fundamental of Environmental Pollution. S.Chand & Co., Ltd.
7. Khan, K.H.; Ahmad, N.; Sial, J.K. and Khan, M.I. 1996. Ground water pollution by heavy metals. Sci. techol. Develop. 14:1-5.
8. Kobya, M.; Demirbas, E.; Senturk, E. and Ince, M. 2005. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 96(13): 1518–1512.
9. Prasad, M. and Freitas, H. 2003.Metal hyperaccumulation in plants—biodiversity prospecting for phytoremediation technology. Electron.j.Biotechnol., 6: 285–321.
10. Regine, H. and Volesky, B. 2000. Biosorption: a solution to pollution. Int. Microbiol. 3: 17-24.
11. Saikaew, W. and Kaewsarn, P. 2009. Cadmium ion removal using biosorbents derived from fruit peel wastes. Songklanakarin J. Sci. Technol. 31 (5): 547-554.
12. Montanher, S.F.; Oliveira, E.A. and Rollenberg, M.C. 2005. Removal of metal ions from aqueous solutions by sorption onto rice bran. J. Hazard. Mater. 117:207-211.
13. Vijayaraaghavan, K.; J.; Jegan, K. P. and Velan, M. 2005. “Batch and Column Removal of Copper from Aqueous Solution using a Brown Marine Alga. J. Chem. Engg., 106: 177 – 184.
14. Volesky B. 2007. Biosorption and me, Water Research, 41: 4017–4029.
Поступила в редакцию 21.05.2013 г.